- Markets & Solutions
- Products
- Capabilities
- Industrial Solutions
- Industrial Solutions Overview
- High Temperature Silicone Seals and Silicone Gaskets
- Silicone Rubber Tubing
- Architectural Building Gaskets
- Silicone Moulding
- Dielectric Silicone Sleeving in Electricity
- Silicone Sponge and Silicone Foam
- Silicone Prototype and Design
- Our Commitment to Quality
- Literature and Downloads
- Quality
- Markets & Solutions
- Products
- Capabilities
- Industrial Solutions
- Industrial Silicone Solutions Overview
- High Temperature Silicone Seals and Silicone Gaskets
- Silicone Rubber Tubing
- Architectural Building Gaskets
- Silicone Moulding
- Dielectric Silicone Sleeving in Electricity
- Silicone Sponge and Silicone Foam
- Silicone Prototype and Design
- Our Commitment to Quality
- Literature and Downloads
- Quality
- Markets & Solutions
- Products
- Capabilities
- Industrial Solutions
- Industrial Silicone Solutions Overview
- High Temperature Silicone Seals and Silicone Gaskets
- Silicone Rubber Tubing
- Architectural Building Gaskets
- Silicone Moulding
- Dielectric Silicone Sleeving in Electricity
- Silicone Sponge and Silicone Foam
- Silicone Prototype and Design
- Our Commitment to Quality
- Literature and Downloads
- Quality
- Markets & Solutions
- Products
- Capabilities
- Industrial Solutions
- Industrial Silicone Solutions Overview
- High Temperature Silicone Seals and Silicone Gaskets
- Silicone Rubber Tubing
- Architectural Building Gaskets
- Silicone Moulding
- Dielectric Silicone Sleeving in Electricity
- Silicone Sponge and Silicone Foam
- Silicone Prototype and Design
- Our Commitment to Quality
- Literature and Downloads
- Quality
- Markets & Solutions
- Products
- Capabilities
- Industrial Solutions
- Industrial Silicone Solutions Overview
- High Temperature Silicone Seals and Silicone Gaskets
- Silicone Rubber Tubing
- Architectural Building Gaskets
- Silicone Moulding
- Dielectric Silicone Sleeving in Electricity
- Silicone Sponge and Silicone Foam
- Silicone Prototype and Design
- Our Commitment to Quality
- Literature and Downloads
- Quality


About
Why Choose Silicone Altimex?

Blue-chip medical and pharmaceutical/biotechnology companies come to Silicone Altimex for medical device components and pharmaceutical assemblies that meet exceptional quality standards. Silicone Altimex offers a full suite of products and capabilities to existing customers and new prospects.
Products:
- Ultra-pure platinum addition silicone
- Platinum pump tubing for high performance pump life
- One-part platinum materials for improved processing repeatability
- Tight tolerance pump tubing
- High performance cardiopulmonary tubing
- Bio-compatible materials meeting USP Class VI, European Pharmacopeia and ISO 10993 standards
- Fully traceable product including batch printing directly onto the product where required
Capabilities:
- Six ISO 14644-1 Class 7 cleanroom facilities
- Routine viable and nonviable monitoring of cleanrooms ensure optimum manufacturing environment
- Laser loop control systems – continuously monitor dimensions and adjust machinery to maintain optimum tolerances
- Complete traceability from raw materials to final packaged product
- State-of-the-art manufacturing equipment with non-contact laser controlled extrusion and inspection release
- Dedicated platinum extrusion cleanroom ensures no contamination from peroxide by-products
- High tolerance capability using continuous feedback loop control extrusion process
- Extensive in-house materials testing and development capabilities
History
Silicone Altimex Limited was established in 1976 as a manufacturer of silicone tubing and other rubber components for several markets in the UK. Since that time, the company has extended its capabilities and evolved into a leading supplier to medical, pharmaceutical and high technology companies worldwide.
Silicone Altimex timeline:
2019
Silicone Altimex spun out into CTS BP portfolio
2015
Silicone Altimex acquired by Q Holding Company
2014
New final operations room and pharma assembly cleanroom added
2012
Specialised Platinum grade ‘SA 900/60 Pump’, a major breakthrough in silicone pump tubing, launched
2009
New purpose built platinum cleanroom facility established at Pasture Road site
2005
Pharmaceutical assembly room established; higher gowning and procedural requirements instituted
Installation of new braiding machine for reinforced pharmaceutical tubing
1998
First LSR (Liquid Silicone Rubber) moulding machine installed in the cleanroom facility to support the medical device market
1997
Second cleanroom installed to manufacture platinum-addition cured products, first for bio pharmaceutical customers and later for other safety-critical components for the aerospace and electronics industries
1994
Development work using platinum cured materials initiated
1979
First cleanroom installed to manufacture tubing and moulded components for the medical market; now also used as an assembly and test area for finished goods
1976
Silicone Altimex founded as a silicone tubing manufacturer for the United Kingdom’s domestic appliance market
Request a quote or learn more
Request a quote or learn more about Silicone Altimex’s extensive services and broad range of capabilities:


Navigate
Silicone Altimex
Contact Us
Global Headquarters
Silicone Altimex
Manufacturing and Engineering Facility
49 Pasture Road
Stapleford, Nottingham, NG9 8HR
0115 949 6890
Additional ways to contact us
Silicone Altimex & Q Holding Locations
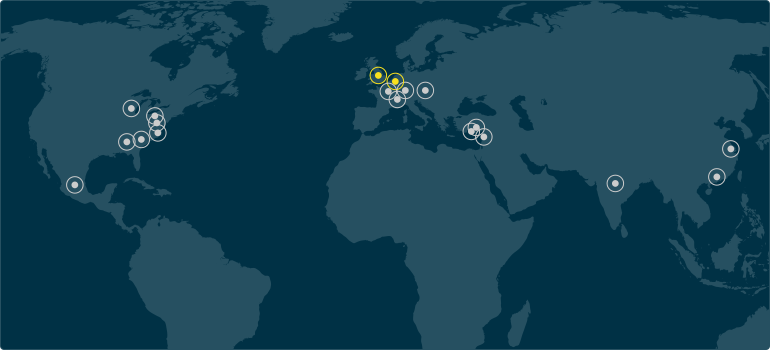 View all Silicone Altimex location details.
View all Silicone Altimex location details. Silicone Altimex Location
Additional Q Holding Company Location
©2024 Silicone Altimex, a SaniSure Company • All Rights Reserved • Terms & Conditions
Track Your Silicone Altimex Shipment
For order tracking in Jasper, GA, contact your QSR customer service representative:
Vikki Stanger
vstanger@qsr-inc.com
706-692-8626
For order tracking in China, contact your QSR customer service representative:
Mary Zheng
mary@qsrchina.com
+ 86-769-836-959-90
For order tracking in Jasper, GA, contact your QSR customer service representative:
Vikki Stanger
vstanger@qsr-inc.com
706-692-8626
For order tracking in China, contact your QSR customer service representative:
Mary Zheng
mary@qsrchina.com
+ 86-769-836-959-90